In follow up to part 1 of this series, we get a lot of questions about stainless steel and its properties. Sometimes the answers are straightforward, and sometimes they might be surprising! Below, we have a few of the common questions we hear:

1) Can stainless steel rust?
Yes, as opposed to what is commonly believed, stainless steel can rust when the environment is too corrosive. The rust will generally appear as pitting (small pinholes on the surface – picture 1 – left) as opposed to uniform corrosion, which is generally the case for carbon steel (picture 2 – right).

2) What are the most common causes of failures for stainless steel?
Throughout the years, experts at CEP have worked on hundreds of failures involving stainless steel. Most commonly, the failures result from one or a combination of the following factors:
Improper surface finish has a very important effect on the corrosion resistance of metals. The smoother the finish the better the corrosion resistance will be. However, a smooth surface is difficult to achieve. Additionally, It is highly reflective and shows scratches, which means the chosen finish is often a compromise between optics and corrosion resistance.
 Picture 3 (right) shows an industrial part with laser engraved numbers. The smooth surface is in good condition, but the engraved portions show significant corrosion, caused by the significantly rougher surface finish.
Picture 3 (right) shows an industrial part with laser engraved numbers. The smooth surface is in good condition, but the engraved portions show significant corrosion, caused by the significantly rougher surface finish.
Wrong choice of alloy: As discussed in Part 1 of this series, dozens of stainless-steel alloys are commercially available. However, they each have different properties, including different levels of corrosion resistance. Cost and availability are significant factors and influence the alloy selected. For example, CEP was involved in a project where a specialized alloy, with a very long lead time, was replaced at the last minute, by a lower grade alloy that was immediately in stock. The machine had to be shut down after only a few months because of catastrophic corrosion, while the expected durability of the originally specified alloy was a few years.
Very corrosive environments: Stainless steels have good corrosion resistance in a lot of common environments. However, they are still subject to rapid degradation when in significant contact with some chemicals, like chlorides, which disrupt the chromium oxide layer that protects the alloy.

A classic example of this phenomenon is the use of cleaners that contain hydrochloric acid. This can result in very rapid pitting of most common stainless-steel grades (picture 4 – left). These products are
used in numerous applications, including masonry cleaner and commercial maintenance. In one project, a dilution error had caused rapid corrosion of a large piece of commercial equipment because the acid ended-up being a lot more concentrated than intended. This led to significant pitting, requiring complete replacement (pictures 5 and 6 – below left and right)


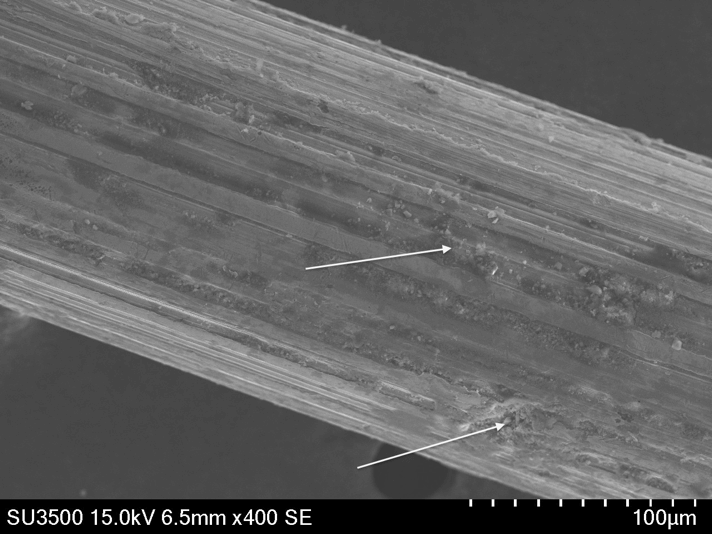 Also, some parts combine several issues like improper surface finish, wrong choice of alloy, or a corrosive environment, making them very likely to fail. For example, some braided flexible connectors combine the use of low-grade stainless steel (200 series) and of wires with significant manufacturing defects (deep longitudinal scratches – picture 7). Even minimal exposure to cleaning products (typically stored under sinks) can create pitting and stress corrosion cracking, leading to a loss of mechanical integrity and leaks (picture 8 – bottom). Thus, losses directly result from the poor-quality wires used by the manufacturer.
Also, some parts combine several issues like improper surface finish, wrong choice of alloy, or a corrosive environment, making them very likely to fail. For example, some braided flexible connectors combine the use of low-grade stainless steel (200 series) and of wires with significant manufacturing defects (deep longitudinal scratches – picture 7). Even minimal exposure to cleaning products (typically stored under sinks) can create pitting and stress corrosion cracking, leading to a loss of mechanical integrity and leaks (picture 8 – bottom). Thus, losses directly result from the poor-quality wires used by the manufacturer.

