Lithium-ion batteries are now an integral part of common objects that we use every day, including smart watches, tools, automobiles, computers, and more. These batteries often have a simple appearance, such as a cylindrical or rectangular shape, and are encased in plastic boxes or rectangular pockets installed and integrated inside electronic devices.
The casings of these batteries hide their design and construction well. As with other types of batteries, they normally consist of an anode and a cathode. In the case of lithium-ion batteries, the anodes are typically made of graphite and the cathodes made with complex oxides. These components are respectively deposited in very thin layers on current collectors, typically made of aluminum for the cathode and copper for the anode. The two sides (anode and cathode) are separated by a plastic film (separator).
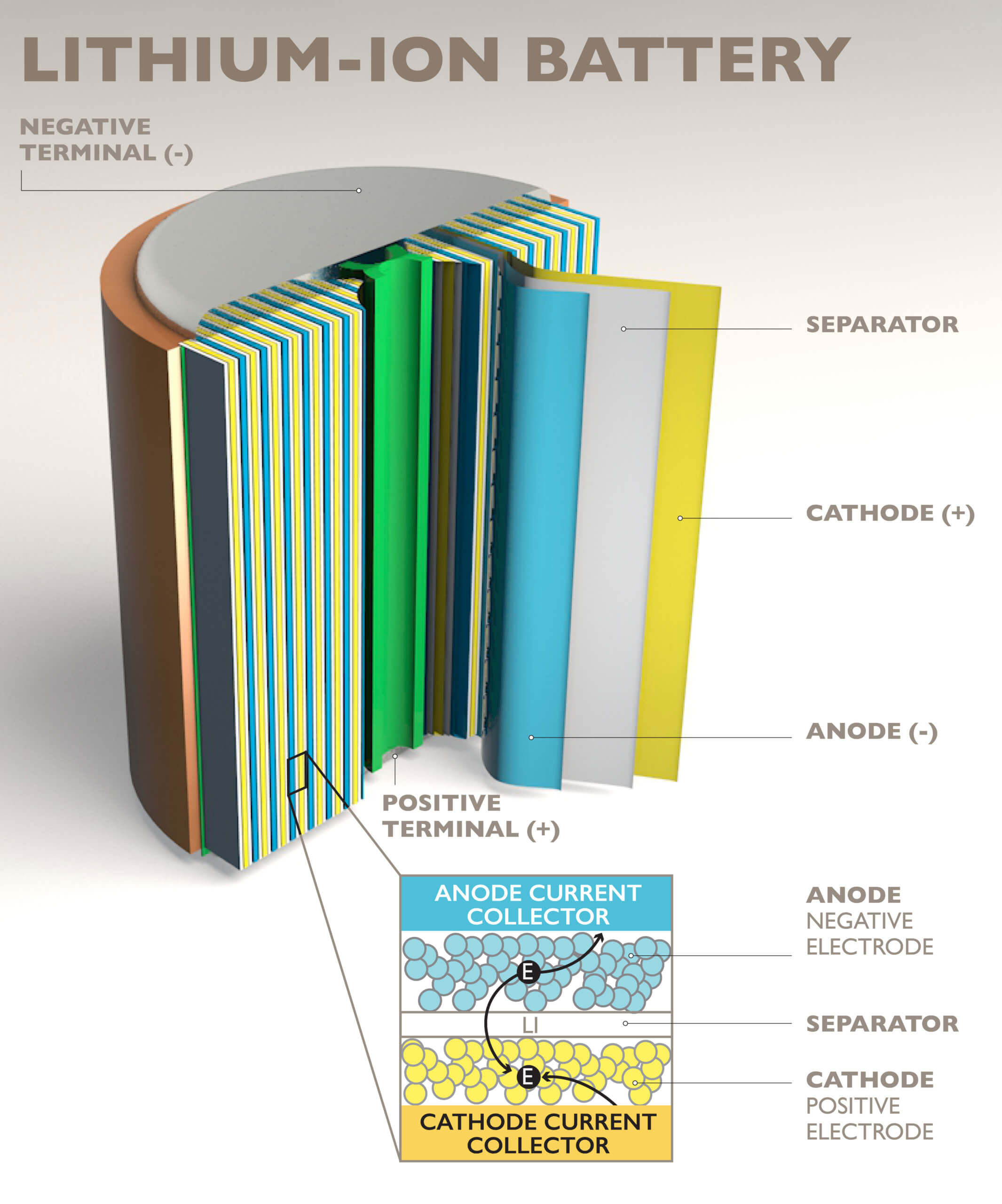
The current collectors and the separator can be superimposed on a rectangular battery or even wound to manufacture a cylindrical element. Lithium ions flow to the anode when charging and to the cathode when discharging (using the battery). This movement of ions is only possible if an electrolyte (liquid or gel) is added to the mix. However, this electrolyte is combustible.
Lithium-ion batteries are very dense assembly, with large energy capacities, containing a highly combustible constituent. Although their manufacturing process is very reliable, some batteries may contain defects. Around 2010, the rate of reported problems with lithium-ion batteries was on the order of 1 in 5 – 10 million produced. These faults rarely generate a catastrophic failure. Current manufacturing processes also tend to be more reliable (for known and established manufacturers). Some electronic protections are also added to battery packs, such as built-in circuitry to avoid overcharging.
Thus, although the failure rate is very low, it is not zero. In addition, various types of damage can occur over the life of a battery. Internal components can be damaged by shocks (with or without puncture of the outer casing), inadequate charging (overcurrent or overvoltage during charging) or discharging problems (continuous heavy usage), wear, use at high temperatures (overheating), etc. Therefore, the condition of batteries during their period of use and at the end of their life can vary greatly from one battery to another. Unfortunately, the state of internal degradation of a battery cannot be characterized by a visual examination of its protective casing. In many cases, the enclosures do not display any clues that could indicate that an immediate failure is about to happen. However, some internal components may be altered, such as the separator film (which may be punctured, altered, or damaged by short circuit).
Lithium-ion batteries can be subject to thermal runaway phenomena which, in some cases, will cause fires. Due to their high energy capacity and internal composition of these batteries, extinguishing fires caused by their failure is also quite difficult. A faulty battery can also initiate thermal runaway on other batteries if they are stored nearby or in direct contact.

Recycling is, for example, an activity that has a certain level of fire risk. Recycling is important but it is necessary to take caution regarding the storage conditions of the batteries. It is important to avoid storing several batteries, including lithium-ion batteries, in combustible and unsealed containers. When they are simply thrown in a box, the failure of a single battery is likely to cause it to ignite and cause significant damage. Mixing several types of batteries is also problematic because traditional batteries can start fires by external shorting. This phenomenon, which should not be overlooked, is also well documented. Consequently, to avoid problems, it is best to store the batteries to be recycled in airtight metal containers, away from combustible objects.
